Abstract
Introduction
Treatment with monoclonal antibodies has revolutionized clinical medicine, especially in the fields of cancer and immunology. One of the oldest antibodies, which is widely used for the treatment of lymphomas and autoimmune diseases, is the anti-CD20 antibody rituximab. In recent years, new antibodies against CD20 have been developed, notably ofatumumab and obinutuzumab. Both antibodies are currently used for the treatment of chronic lymphocytic leukemia (CLL), with a possible clinical advantage of obinutuzumab over rituximab. In addition to CLL, obinutuzumab was recently approved for the treatment of relapsed/refractory follicular lymphoma in patients who had previously received a rituximab-containing regimen.
An important mechanism of action of therapeutic monoclonal antibodies is activation of immune cells via Fc receptors (FcγRs). Surprisingly, however, the interactions of various therapeutic antibodies with various Fc receptors have not been fully elucidated. Moreover, the available data on the interaction of different anti-CD20 antibodies and human Fc receptors is based on affinity measurements, mainly surface plasmon resonance (SPR), and not on functional assays. Although primary immune cells can help us gain information regarding the activation of Fc receptors, these cells are difficult to handle and usually express several Fc receptors.
Methods
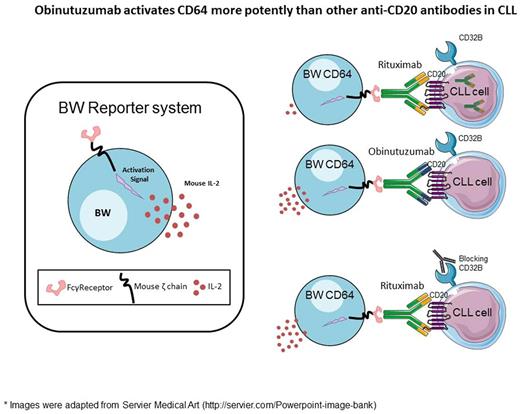
Here we established a functional assay to study how different anti-CD20 antibodies activate different human Fc receptors. To this aim, we stably transfected mouse BW5147 cells (BW cells) with chimeric FcγRs which included the extracellular part of a given FcγR fused to the transmembrane and cytoplasmic segments of the mouse CD3ζ chain. Activation of a specific FcγR results in secretion of mouse interleukin-2 (mIL-2) which can be detected by enzyme-linked immunosorbent assay (ELISA). We prepared BW cells which express the following human FcγR-CD3ζ chimeras: CD16A (FcγRIIIA, low and high affinity variants 158F and 158V respectively), CD32A (FcγRIIA, low and high affinity variants, 131R and 131H respectively), and CD64 (FcγRI). The advantages of this system are that it is a functional assay, easy to use, sensitive (was used to detect anti-viral antibodies in patients) and enables to study the activation of a single Fc receptor. Additionally, the activation of a specific Fc receptor by this reporter system is studied in the context of the natural ligand of the antibody, which better reflects the activity of the antibody in vivo .
We assessed the activation of Fc receptors by different anti-CD20 antibodies which were incubated with chronic lymphocytic leukemia (CLL) cells obtained from patients.
Results
Using this functional assay we corroborated previous reports regarding the higher affinity of obinutuzumab to CD16A. Importantly, we discovered that obinutuzumab activates CD64 significantly more efficiently than rituximab or ofatumumab. We show that the advantage of obinutuzumab in activating CD64 translates into better elimination of CLL cells by primary macrophages. Mechanistically, we found that CD64 is activated more potently by obinutuzumab since obinutuzumab does not internalize when bound to its target protein CD20, in contrast to rituximab and ofatumumab. We show that the internalization of rituximab and ofatumumab is mediated by binding to the FcγR CD32B, which is expressed on the surface of CLL cells. Indeed, blocking of CD32B led to increased activation of CD64 by rituximab and ofatumumab.
Discussion
Using a functional assay we found that obinutuzumab activates CD64 more potently than other anti-CD20 antibodies. Is it possible that this advantage is relevant only to tumor cells with high expression of CD32B. This could be applied clinically by incorporating obinutuzumab instead of rituximab in certain lymphoproliferative diseases (e.g., CLL) or specifically in patients with high expression of CD32B. Furthermore, the BW reporter assay can be applied to other therapeutic antibodies and may assist in developing antibodies with improved immunological properties.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal